Ml of tap water into a barium hydroxide and hydrochloric acid net ionic equation clean beaker. The balanced equation for this reaction is.
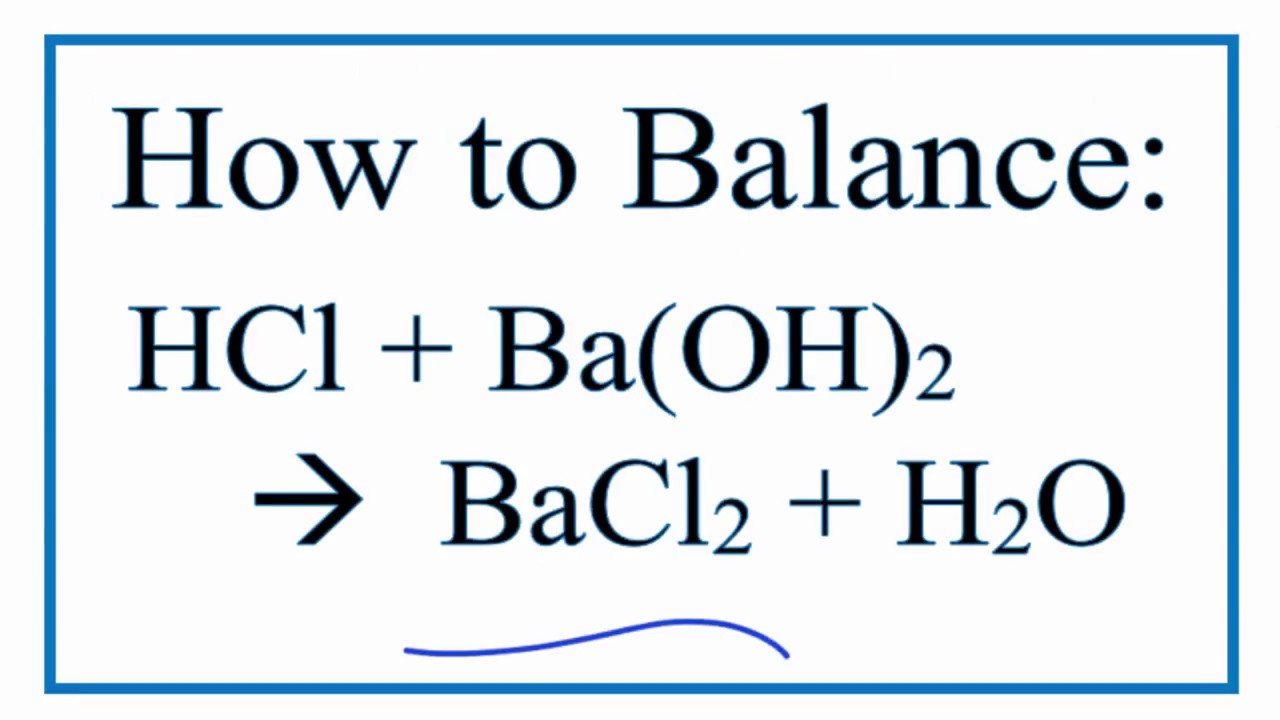
How To Balance Hcl Ba Oh 2 Bacl2 H2o Hydrochloric Acid Plus Barium Hydroxide Youtube
When barium hydroxide is titrated with hydrochloric acid two molecules of hydrochloric acid combine with one molecule of barium hydroxide to produce one molecule of barium chloride and two molecules of water.

. Ba OH2 is a strong base while HCl is a strong acid. Balance the complete ionic equation when aqueous barium hydroxide reacts with aqueous hydrochloric acid asked Jun 26 2017 in Chemistry by Champoo ANSWER. Solid zinc sulfideoxygen gas longrightarrow solid zinc oxide sulfur dioxide gas.
This forms a salt and water. Mole s of barium chloride and mole s of water. Write a balanced equation for the reaction below.
Find step-by-step Chemistry solutions and your answer to the following textbook question. 2 HCl aq Ba OH2 aq BaCl2 aq 2 H20 1 We can interpret this to mean. Additionally what are the products in the molecular equation for the complete neutralization reaction of aqueous barium hydroxide and nitric acid.
When barium hydroxide is titrated with hydrochloric acid two molecules of hydrochloric acid combine with one molecule of barium hydroxide to produce one molecule of barium chloride and two molecules of water. 2HClaq BaOH2 aq BaCl2aq 2H2 01 If 4 moles of barium hydroxide react The reaction consumes moles of hydrochloric acid. Include symbols for physical states in the equation.
The balanced equation for this reaction is. Equation for the reaction that occurs when aqueous solutions of barium hydroxide and hydrochloric acid gives barium chloride water. When hydrochloric acid reacts with barium hydroxide barium chloride and water are produced.
2 moles of hydrochloric acid and mole s of barium hydroxide React to produce. What is the balanced equation for the reaction of hydrochloric acid and barium hydroxide. Click hereto get an answer to your question Balance the chemical equation by including the physical states of the substances for the following reactionsa Barium chloride and sodium sulphate aqueous solutions react to give insoluble Barium sulphate and an aqueous solution of sodium chlorideb Sodium hydroxide reacts with hydrochloric acid to produce sodium.
Similarly what are the products in the molecular equation for the. Since there is no overall transfer of electrons this cant be a redox reaction and that means activities wont matter here. How to Balance HCl Ba OH2 BaCl2 H2O Hydrochloric Acid plus Barium Hydroxide - YouTube.
2HCl BaOH2. The balanced equation for this reaction is. 2HCl aq Ba OH2 aq BaCl2 aq 2H2 0 1 If 4 moles of barium hydroxide react The reaction consumes moles of hydrochloric acid.
Barium hydroxide is also a strong base strong electroyte so it also dissociates completely. What is the balanced equation for the reaction of hydrochloric acid and barium hydroxide. First write a balanced molecular equation with states.
K O H a q H N O 3 a q H 2 O l K N O 3 a q b Hydrochloric acid is a strong acid meaning it is a strong electrolyte so it dissociates completely when in an aqueous solution. A balanced chemical equation follows the. A Ba 2HCl BaC l 2 2H B Ba 2HCl BaC l 2 H 2 C Ba 2HCl Ba H 2 Cl D Ba HCl BaHCl Verified 1614k views Hint.
Sodium Hydrogen Sulfite reacts with hydrochloric acid to produce sulfur dioxide gas water and sodium chloride NaHSO3 HCl ----- SO2 H2O NaCl 2. 2HCl aq Ba OH2 aq BaCl2 aq 2H2 0 1 If 4 moles of barium hydroxide react The reaction consumes moles of hydrochloric acid. The reaction produces moles of barium chloride and moles of water.
When hydrochloric acid reacts with barium hydroxide barium chloride and water are produced. When two solutions of hydrochloric gives. The balanced chemical equation for the reaction between hydrochloric acid and barium hydroxide is.
The reaction 2K s Br2 l 2KBr s is a n ______________ reaction. CaCO3 s 2HCl aq CaCl2 aq H2O l CO2 g Now write a balanced ionic equation. Aqueous hydrochloric acid aqueous barium hydroxide.
Ba OH2 is a strong base while HCl is a strong acid. 2HCl BaOH2. The equation for this reaction is Ba OH2 2 HCl BaCl2 2 H2O.
Barium hydroxide and hydrochloric acid net ionic equation There are three main steps for writing the net ionic equation for Mg HCl MgCl2 H2 Magnesium Hydrochloric acid. Prepare aminonaphtholsulfonic acid TS fresh on the day of use by dissolving 1. Sodium Hydroxide Hydrochloric Acid - Balanced Molecular Equation Complete and Net Ionic Equation.
2HClaq BaOH2aq BaCl aq 2H O1 If 6 moles of hydrochloric acid react The reaction consumes moles of barium hydroxide. The equation for this reaction is Ba OH2 2 HCl BaCl2 2 H2O. Interactive video lesson plan for.
Write the chemical equation that relates to each of the following word equations. Ba2 aq 2OH- aq 2H aq 2Cl- aq 2H2O l Ba2 aq 2Cl- aq 34. OH- g -- H_2O l it.
Barium hydroxide reacts with hydrochloric acid to form barium chloride and water. Hydrochloric acid aqueous sodium hydroxide 7. BaCl2 2H2O What is the equation of barium sulphate with hydrochloric acid.
Write the balanced chemical equation when barium reacts with hydrochloric acid. Metal hydroxides are ionic compounds so they are also strong electrolytes. The balanced equation for this reaction is.

Solved When Hydrochloric Acid Reacts With Barium Hydroxide Chegg Com
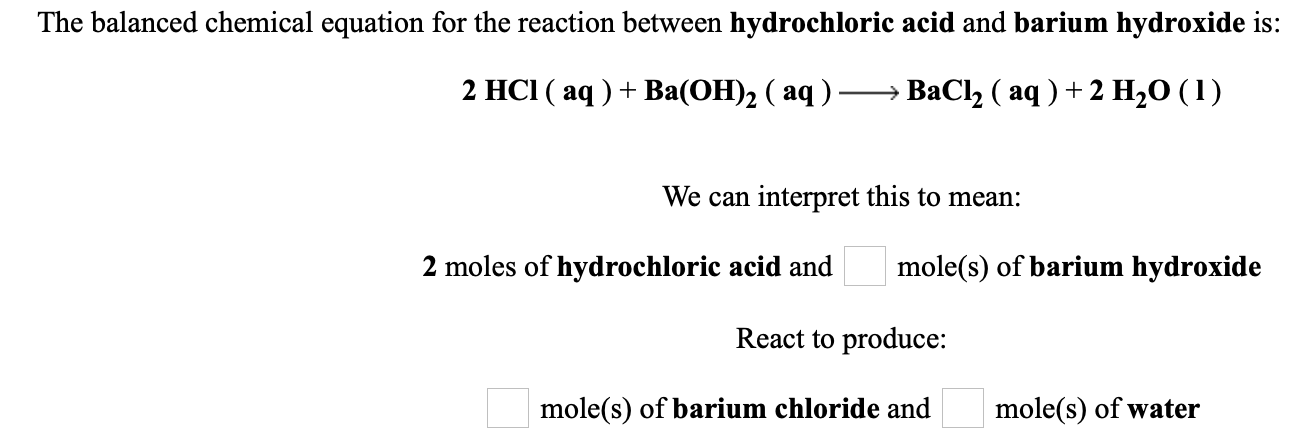
Solved The Balanced Chemical Equation For The Reaction Chegg Com

0 Comments